Clinical trials: EMA launches public consultation on draft guideline for using computerized systems and electronic data - Portolano Cavallo

Data Management for Pharmaceutical Trials Michael A. Kohn, MD, MPP (Acknowledgment: Susanne Prokscha) - ppt download

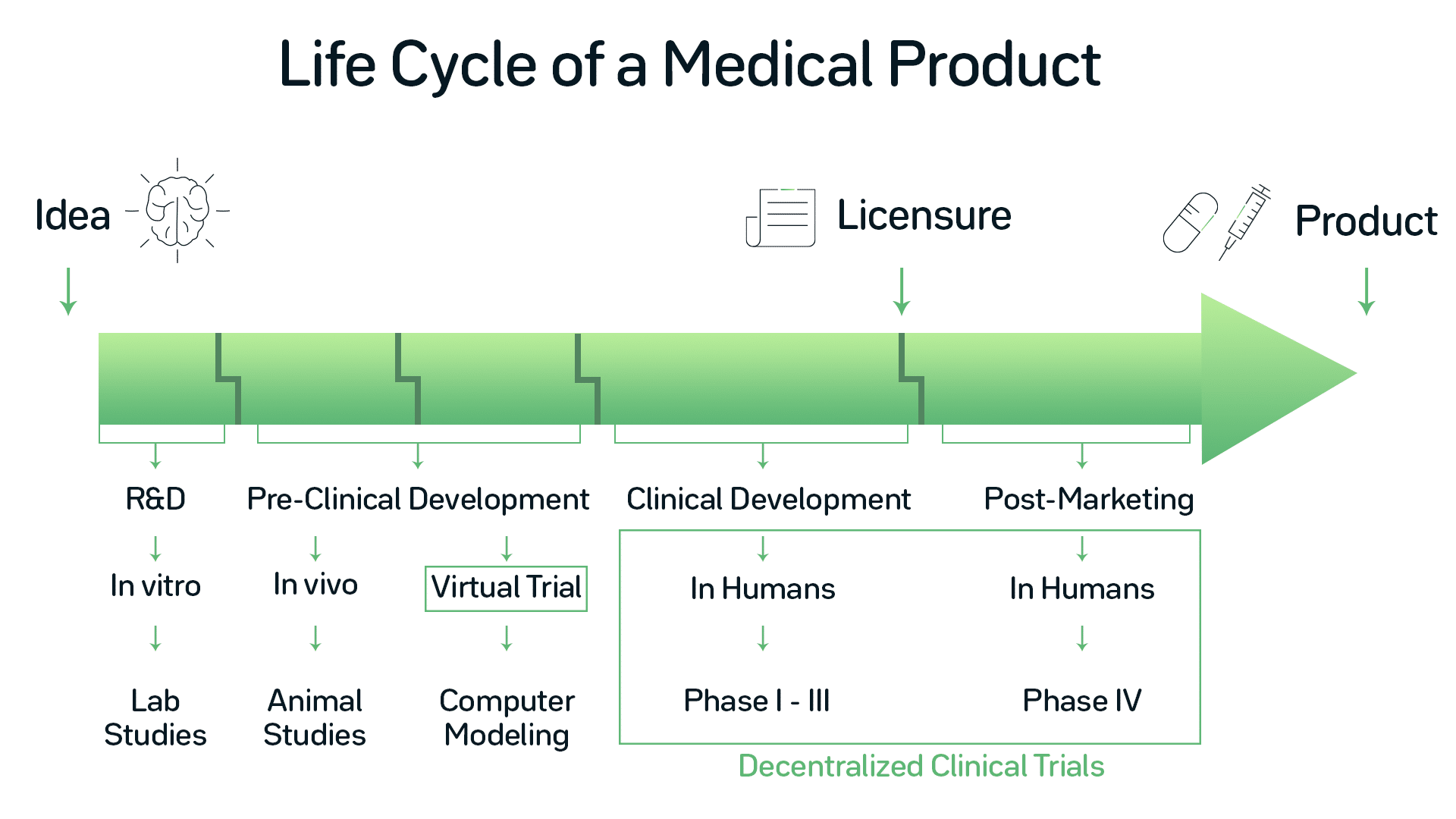
Incorporating Site-less Clinical Trials Into Drug Development: A Framework for Action - ScienceDirect
An interactive retrieval system for clinical trial studies with context-dependent protocol elements | PLOS ONE

PPT - Session 6: Data Integrity and Inspection of e-Clinical Computerized Systems PowerPoint Presentation - ID:1597364

PDF) Guidance for Industry Computerized Systems Used in Clinical Investigations | Amutha V - Academia.edu

Guidance Document: Part C, Division 5 of the Food and Drug Regulations “Drugs for Clinical Trials Involving Human Subjects” (GUI-0100) - Canada.ca

Session 6: Data Integrity and Inspection of e-Clinical Computerized Systems May 15, 2011 | Beijing, China Kim Nitahara Principal Consultant and CEO META. - ppt download
![Computerized Systems Used in Clinical Investigations, Guidance for Industry, 2007, US FDA [和訳付] | Manualzz Computerized Systems Used in Clinical Investigations, Guidance for Industry, 2007, US FDA [和訳付] | Manualzz](https://s1.manualzz.com/store/data/009789475_1-cf6f9033de9e2c7d1122b68284a7b5dd.png)
Computerized Systems Used in Clinical Investigations, Guidance for Industry, 2007, US FDA [和訳付] | Manualzz